

It is estimated that slightly more than three-quarters of all naturally occurring elements exist as a mixture of isotopes the average isotopic mass of an isotopic mixture for an element in a specific environment on Earth is used to calculate the element’s standard atomic weight.
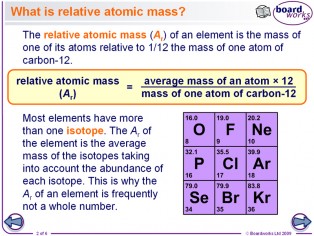
In an element in a specific environment on Earth, its standard atomic weight is determined by the average isotopic mass of an isotopic mixture of that element’s atoms. Isotopes are atoms that have the same atomic number but have different neutron numbers, and so have different mass numbers from one another. It is the energy level around the nucleus in a neutral atom that is equal to the number of protons in shells, in which case the atom is considered neutral. It is equal to the amount of protons in an element’s nucleus that determines the element’s atomic number.

Protons, neutrons, and electrons are found in every atom, with the exception of the common form of hydrogen. Innumerable combinations are possible, resulting in the formation of many compounds. Because Aristotle’s beliefs were widely accepted throughout Europe for about 2000 years, the concept of atoms was put on hold for several centuries.Ītoms are the fundamental constituents of matter. The famous Greek philosopher Aristotle, on the other hand, did not share their sentiments. The theory that the cosmos is made up of small indivisible pieces, which they termed atoms, was first proposed by a Greek philosopher named Leucippus and his pupil Demokritos around 2500 years ago.


 0 kommentar(er)
0 kommentar(er)
